I’m sure you’ve heard the term “soil pH” thrown around before when referring to the soil in the landscape or regarding what pH a particular plant prefers. But what is soil pH really? Well, the pH of the soil is a measure of acidity or alkalinity. It basically indicates how ‘sour or sweet’ the soil is for lack of a better word.
The pH Scale
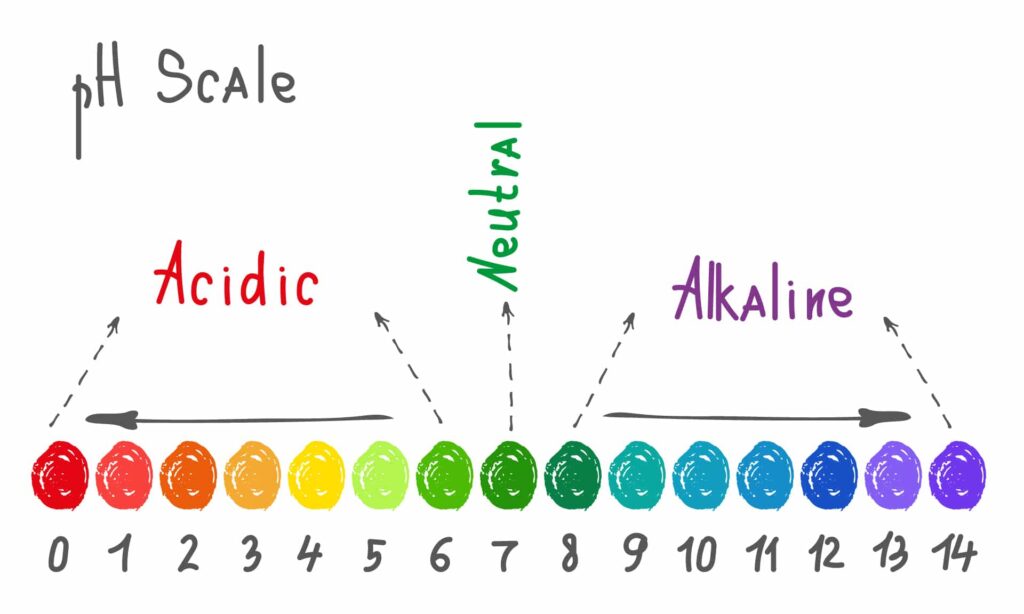
The pH scale ranges from 0 (extremely acidic) to 14 (extremely alkaline), with 7 being right in the middle and thus neutral. Values below 7 indicate an acid soil, and values above 7, alkaline soil. Because the scale is logarithmic (a convenient way of expressing very large numbers), a pH change by 1 unit means it is 10 times more acidic or alkaline.
The pH level affects growth in that it affects the plants’ nutrient availability and how a plant absorbs specific nutrients. For one somewhat complicated example, pH levels below 6 (leaning acidic) cause the solubility of phosphoric acid, calcium, and magnesium to drop, whereas levels between 3 and 5 (even more acidic) plus temperatures above 78 degrees F. can encourage the development of a variety of fungal diseases. There is a whole world of plants preferring ranges of pH and there are ways to alter it.
Other Factors That Affect pH
Another contributing factor to pH levels is sunlight or lack thereof. Daylight/sunlight allows photosynthesis which produces hydrogen ions, causing the nutrient acidity to increase (lowering the pH in the soil). However, at dusk photosynthesis stops and the plants increase their rate of respiration, and this coupled with the respiration of microorganisms and the decomposition of organic matter uses up the hydrogen ions, so the acidity of the soil tends to decrease (pH rises).
Other differentiating variables related to light availability in relation to the soil pH levels are: in low light (overcast days) plants take up more potassium and phosphorous from the nutrient solution so the acidity increases (pH drops). In strong, intense light (clear sunny days) however, plants take up more nitrogen from the nutrient solution causing the acidity in the soil to decrease (pH to rise). Now some extremes in pH levels can result in the precipitation of certain nutrients. For plant roots to be able to absorb nutrients, the nutrients must be dissolved in the solution. The process of precipitation (the reverse of dissolving) results in the formation of solids in the nutrient solution, therefore making the nutrients unavailable to the plants.
Some ways of avoiding the precipitation of the nutrient solution are making sure plants get sufficient water, nutrition, and sunlight. This will build up a natural resistance against pests and diseases. It, therefore, pays to keep the soil pH around neutral to avoid certain nutrient deficiencies, which will weaken the plants and make them more vulnerable to pest and disease attacks.
What pH Do Plants Prefer?
Most plants prefer neutral soil, however, some are suited to other pH levels. Here are some examples of different ornamentals that prefer a pH level of around 4.5 to 6.0 (leaning acidic):
- Azaleas and Rhododendrons
- Camellia
- Gardenias
- Holly
- Macrophyllla Hydrangeas
- Blueberries,
- Magnolia
- Cranberries,
- Potatoes.
Here are some plants that can handle a soil pH of around 7.0 to 8.0 (alkaline)
- Cacti
- Gerbera
- Hibiscus
- Geranium
- Ivy
- Poinsettia
- Viburnum
- Melon
- Beetroot
- Leek and Spinach
- Buddleia
- Deutzia
- Forsythia
- Some Hydrangea
- Lilac
- Weigela
What Does it All Mean?
To review, soil pH determines the nutrient availability to plants. Some nutrients become ‘tied up’ in the soil at certain levels. For example, acid soils can lead to deficiencies of phosphorus, calcium, magnesium, and molybdenum, as well as toxic levels of manganese and aluminum, while alkaline soils may lead to deficiencies in iron, manganese, boron, copper, and zinc.
Consider utilizing a pH soil tester or completing a soil test to determine the status of your soil and then optimize it. Many gardeners choose to use products like Soil Acidifiers like Espomas Organic Soil Acidifier or Aluminum Sulfate that lower pH or Espoma’s Organic Garden Lime to raise pH. These products and many more are available at our Garden Center in Des Plaines. We are here to help answer any questions and would love to talk and research the pH needs of your favorite plants.










